Introduction: Bispecific antibodies (BisAb) are effective therapies for relapsed myeloma yet associated with high risk of potentially severe infections. A prior study from our center did not associate any baseline characteristics with 90-day risk of infection. In clinical practice patients are already receiving sequential immune effector cell therapy (IET; BisAb or CAR T) it remains unclear whether this compounds the infectious risk. Herein, we examined whether a prior T-cell redirecting therapy predisposes to increased risk of infection during subsequent BisAb.
Methods: This is a single-center, retrospective analysis of patients receiving BisAb therapy targeting B-cell maturation antigen (BCMA), G protein-coupled receptor family C group 5 member D (GPRC5D), or Fc receptor-homolog 5 (FCRH5) between January 2017 and February 2023. BisAb treatment was considered a treatment event (TE) such that if a patient received two different BisAbs, this would be considered two separate TEs. We obtained clinical data from before BisAb therapy start date: prior CAR T therapy, prior BisAb therapy, and infections 90 days before TE). Infection data during the full duration of BisAb therapy was also collected from the time of the first priming dose until 30 days after the last BisAb dose. For follow up for infections, we included any TE with at least one target dose of BisAb. We utilized two-sample proportion tests to analyze the differences in rates of infections between the BCMA and non-BCMA groups.
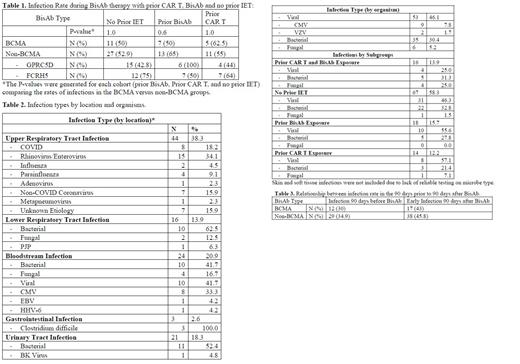
Results: 91 patients and 123 TE episodes were identified. The TE episodes consisted of 40 with BCMA-directed therapy, 48 with GPRC5D-directed therapy, and 35 with FcRH5-directed therapy. Of these 123 treatment episodes, 26 TEs had 1 prior CAR T and 2 TEs had 2 prior CAR T and 28 TEs had received 1 prior BisAb and 6 TEs had received 2 prior BisAb. The most common infections were viral (46.1%) followed by bacterial (30.4%) and fungal (5.2%).
We evaluated the relationship between prior IET (either CAR T or BisAb therapy) and incidence of infections during and up to 30 days BisAb (Table 1). In TEs with at least 1 prior CAR T the incidence of infections was: 62.5% (BCMA), 44% (GPRC5D) and 64% (FCRH5). For BCMA vs non-BCMA, the incidence of infections was 62.5% vs 55% (p=1.0). In TEs with at least 1 prior BisAb, the incidence of infections following subsequent BisAB was 50% (BCMA), 100% (GPRC5D) and 50% (FCRH5). For BCMA vs non-BCMA, the incidence of infections was 50% vs 65% (p=0.6). In TEs without prior IET, the incidence of infections was 50% (BCMA), 42.8% (GPRC5D), 75% (FCRH5). For BCMA vs non-BCMA, the incidence of infections was 50% VS 52.9% (p=1.0). On comparing these cohorts, we found no significant differences between the BCMA and non-BCMA BisAb therapy for following groups: prior BiSAb, prior CAR T, and the group without prior IET.
We evaluated TEs with infections in the 90 days prior to BisAb and their relationship to infections during the first 90 days following BisAb therapy. The infection rate in the 90 days following BisAb therapy increased in TEs with earlier infection in the 90 days before BisAb priming dose from 30% to 43% for BCMA and 34.9% to 45.9% for non-BCMA BisAb.
Conclusions: In this retrospective study we found similar rates of infection between groups with and without prior IET exposure. In patients who had infections during the 90 days before BisAb therapy, there was an increase in infection rate from baseline of 13% for BCMA BisAb and 11% from non-BCMA BisAb in the first 90 days after BisAb therapy. This is the largest cohort to report real-world data on infections following BisAb therapy after prior IET.
Disclosures
Goldsmith:Adaptive Biotechnologies: Speakers Bureau; Janssen Pharmaceuticals: Speakers Bureau; Janssen Pharmaceuticals: Consultancy; Sanofi-Genzyme: Consultancy; Wugen Inc.: Consultancy; Oncovalent: Consultancy; Bristol Myers Squibb: Research Funding. Rosenzweig:Janssen: Other: Grant support, Speakers Bureau. Krishnan:Janssen Biotech Inc: Other: Contracted Research; Amgen Inc, Bristol-Myers Squibb Company, Takeda Pharmaceuticals USA Inc: Other: Speakers Bureau; Adaptive Biotechnologies Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline, Regeneron Pharmaceuticals Inc, Sanofi Genzyme: Other: Consulting Agreements; Bristol-Myers Squibb Company: Other: Stock Options/Ownership-Public Company; Sutro Biopharma: Other: Advisory Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal